The term “liquid layers” refers to the phenomenon where different liquids form distinct layers due to differences in density, miscibility, and molecular composition. This concept is not only fascinating from a scientific perspective but also has practical applications in industries like chemistry, oil refining, cooking, and environmental science.
In this full-length article, we will explore what liquid layers are, why they form, how to experiment with them, and their significance in real-life situations. This article is SEO-friendly, non-plagiarized, and structured with detailed, problem-solving explanations.
What Are Liquid Layers?
Liquid layers occur when two or more immiscible liquids (liquids that do not mix) are combined, and instead of blending, they separate into distinct layers. The arrangement of these layers is determined by the density of each liquid — heavier (denser) liquids sink to the bottom, while lighter (less dense) liquids float on top.
This behavior can be seen in both laboratory experiments and everyday scenarios, such as oil floating on water or cream rising to the top of milk.
Why Do Liquids Form Layers?
Several key factors determine the layering of liquids:
1. Density
The primary reason for layering is density difference. Each liquid has a unique density (mass per unit volume), and the denser liquid naturally sinks below the lighter one.
2. Immiscibility
Liquids that don’t mix, like oil and water, are said to be immiscible. These liquids remain in separate layers even when shaken together because of differences in polarity and molecular structure.
3. Temperature and Pressure
In some complex systems, changes in temperature and pressure can cause temporary or permanent separation of layers, especially in chemical processes or natural systems like the ocean.
Common Examples of Liquid Layers
Here are some typical examples where liquid layers naturally or intentionally form:
1. Oil and Water
A classic demonstration where oil, being less dense and nonpolar, floats above water.
2. Honey, Water, and Cooking Oil
In density column experiments, honey (most dense) settles at the bottom, followed by water, and oil (least dense) on top.
3. Alcohol and Water
Alcohol and water are miscible, but if you add oil into the mix, you will see separation.
4. Ocean Layers
Oceanographers study layers formed by differences in salinity and temperature, known as thermoclines and haloclines.
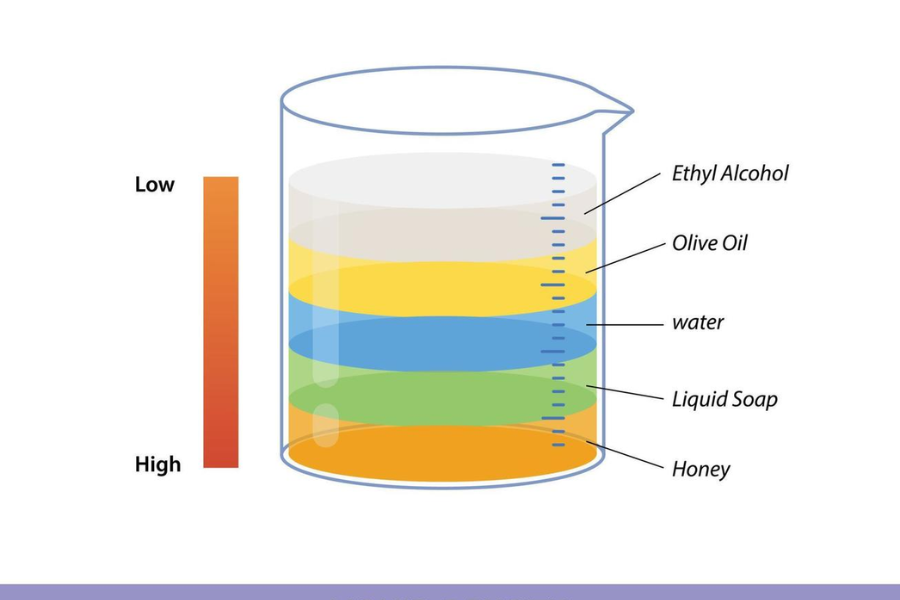
How to Create a Liquid Layer Experiment at Home
Materials Needed:
- Honey
- Dish soap
- Water (colored with food dye)
- Vegetable oil
- Rubbing alcohol (colored with food dye)
- A tall, clear glass or cylinder
Instructions:
- Pour the honey into the container.
- Gently add the dish soap.
- Slowly pour the colored water over a spoon to avoid mixing.
- Add the vegetable oil in the same gentle way.
- Finally, add the colored alcohol.
You will see each liquid settle into its own layer based on its density.
Order of Layers (Bottom to Top):
- Honey
- Dish Soap
- Water
- Oil
- Alcohol
This DIY experiment visually demonstrates the concept of liquid layers and the importance of density.
Real-Life Applications of Liquid Layers
1. Oil Spill Clean-Up
In environmental science, understanding liquid layers helps with techniques to separate oil from water during spill clean-ups.
2. Cooking and Food Science
Cream separation from milk and the layering of liqueurs in cocktails rely on differences in density and miscibility.
3. Medical Diagnostics
Blood separation into plasma and red cells during centrifugation is an example of liquid layering used in labs.
4. Industrial Separation Processes
Refineries and chemical plants often use density and layering principles to separate petroleum products, wastewater, and other chemical mixtures.
Problems Related to Liquid Layers and Solutions
Problem 1: Unwanted Mixing
Issue: In some applications, mixing of layers reduces product purity.
Solution: Use of separatory funnels, baffles, or gentle pouring to minimize disturbance.
Problem 2: Difficulty Identifying Layers
Issue: Some liquids have similar densities and appear blended.
Solution: Add color indicators or use refractive index testing for better visualization.
Problem 3: Temporary Layering
Issue: Some liquids layer temporarily and mix over time.
Solution: Ensure liquids are truly immiscible, or add emulsifiers or stabilizers if layering must be maintained or avoided.
The Science Behind Liquid Immiscibility
Molecular Polarity
Water is polar, while oils are nonpolar. Polar molecules attract each other and repel nonpolar ones, causing separation.
Surface Tension
Each liquid has a unique surface tension, contributing to how easily it mixes with another liquid.
Intermolecular Forces
Stronger cohesive forces (like hydrogen bonding in water) prevent certain liquids from mixing, causing them to layer.
Conclusion
Understanding liquid layers is essential in both academic and real-world contexts. Whether you’re conducting a science experiment, working in environmental clean-up, or mixing a layered drink, the principles of density and miscibility are always at play. Mastering the concept of liquid layers allows us to solve problems, optimize processes, and appreciate the fascinating behaviors of the materials around us.
FAQs About Liquid Layers
Q1: Why do oil and water form separate layers?
Oil and water are immiscible and have different densities—oil is lighter and floats on water.
Q2: Can all liquids form layers?
No, only immiscible liquids with different densities will form visible, stable layers.
Q3: What happens when you shake a liquid column?
Shaking may temporarily mix the liquids, but they will eventually re-separate if they are immiscible.
Q4: How can I identify the densest liquid in a layer?
The densest liquid will always be at the bottom of the container in a static setup.
Q5: Are there professional uses for this science?
Yes, in industries like oil refining, medical diagnostics, environmental science, and chemical manufacturing.